Nothing unusual jumps out upon meeting Evelyn, a bubbly almost-3-year-old with red curls—except that she should not be here, chatting with a visitor in her family’s living room, twirling in her tights to the Pharrell Williams song “Happy.”
Evelyn’s older sister Josephine had spinal muscular atrophy type 1 (SMA1), a genetic disease that gradually paralyzes babies. She died at 15 months. Evelyn was an unexpected pregnancy, but her parents decided to have the baby despite one-in-four odds of a second tragedy.
Soon after Evelyn was born in December 2014, they were devastated to learn from genetic testing that she, too, had SMA1. “We knew what we were dealing with: We’ll love her for as long as we can,” says her father, Milan Villarreal. But that same night, frantically searching the internet, they learned about a clinical trial in Ohio and sent an email. At 8 weeks old, Evelyn received a gene therapy treatment that gave her body a crucial missing protein.
And now here she is, not so different from any healthy toddler. Although she has weak thighs and can’t run normally or jump, she can walk quickly, dance, trace letters, toss foam blocks, carry a small chair, and climb onto her mother Elena’s lap. After the heartbreak of losing their first baby, the Villarreals have watched in amazement as Evelyn has crawled, walked, and talked. “It was just a miracle. Every milestone was like a celebration. We opened a bottle of wine for every little thing she did,” Milan says.

MIKE SHANAHAN
The results of the trial Evelyn participated in have blown away gene therapy researchers, too, marking one of the once-troubled field’s most dramatic successes yet. All 15 babies treated for SMA1, expected to die by age 2, are alive at 20 months or older, and most can sit up, according to a report this week in The New England Journal of Medicine (NEJM). Like Evelyn, one boy is walking. Although a drug recently approved for SMA1 has achieved similar effects, it must be injected into the spine every 4 months. The gene therapy is intended as a one-time treatment, and it is simply infused into a vein. “I’ve never seen an effect [of gene therapy] that good in a lethal disease,” says neurologist Jerry Mendell of Nationwide Children’s Hospital in Columbus, who led the recent trial.
The news adds to the rising fortunes of gene therapy. A treatment for a genetic form of blindness, endorsed last month by a U.S. Food and Drug Administration (FDA) advisory panel, is on track to be the first gene therapy in the United States approved for an inherited disease. Last month, a team reported in NEJM that giving boys a transplant of their own genetically modified stem cells could halt a devastating brain disease. A gene therapy on the market in Europe cured kids with a life-threatening immune disorder, and others in development helped hemophiliacs go off blood-clotting drugs.
But the SMA1 study breaks ground because it demonstrates the power of a new vector, a virus carrying a therapeutic gene that, infused into a vein, can carry its cargo to the central nervous system, across the so-called blood-brain barrier. In the past, efforts to treat neurological diseases with gene therapy often had lackluster results, and delivering the vector sometimes required drilling holes in a patient’s skull. Now, scientists have an undisputed success delivering gene therapy into the central nervous system. The results also allay fears that flooding the bloodstream with virus could lead to another tragedy like the death of a teenager in a gene therapy trial 18 years ago, which threw the field into disarray.
The new treatment’s apparent safety and success is emboldening other researchers to use gene therapy delivered into a vein or the spine to treat rare childhood neurological and muscular diseases, and even common adult disorders such as Parkinson’s disease. “It opens up a whole new concept to a field that has been very cautious,” says Federico Mingozzi, who recently left the French nonprofit Généthon to become chief scientific officer of the gene therapy company Spark Therapeutics in Philadelphia, Pennsylvania.
“People are going to look back and see this as a milestone in a new type of medicine that’s going to have broad implications for lots and lots of diseases,” adds gene therapy researcher Steven Gray of the University of North Carolina (UNC) in Chapel Hill.
At first, when animals were the patients, treating neurological diseases with gene therapy seemed easy. In the 1990s, researchers injected the brains of newborn mice engineered to have certain metabolic disorders with viral vectors carrying a missing gene. The outcome was “shocking, it was so good,” recalls Mark Sands of Washington University School of Medicine in St. Louis in Missouri. But a mouse brain is tiny, he adds. “The challenge was how you scale up from a mouse brain that weighs half a gram to a child’s brain that weights 1000 grams. That’s a 2000-fold difference in scale. How do you make that work?”
It turned out researchers couldn’t. In 1996, a team injected fat particles containing a curative gene into the skulls of two children who had a brain disorder called Canavan disease. The research was controversial, and it did not help the patients. Some of the same researchers later treated 13 more Canavan patients by boring six holes into their skulls and injecting a vector fashioned from an apparently harmless virus known as an adeno-associated virus (AAV); a similar AAV trial took place for another severe childhood genetic brain disorder, Batten disease.
Neither treatment did much to slow disease, however. Researchers in one trial with Taiwanese children who were severely disabled from a deficiency of two brain chemicals did report significant benefits. But results for other diseases have been equivocal. Monkey studies have suggested that the AAV vector did not diffuse far from the injection sites, so not enough brain cells likely received the new gene. “They were heroic efforts in many ways, but the technology wasn’t ideal,” Gray says.
An entirely different approach yielded the only solid successes in neurological disease. Researchers took blood stem cells from patients, used modified HIV to stitch in a new gene, and infused the cells back into the patients. Some of the cells then migrated to the brain and formed neuron-supporting cells called glia that produced a needed protein. In 15 boys, according to a study published last month in NEJM, this “ex vivo” gene therapy stopped a fatal disease called adrenoleukodystrophy (ALD), which destroys the myelin sheath around neurons. Italian gene therapy researchers have reported comparable results for a similar treatment given to young patients with the brain disease metachromatic leukodystrophy.
But ex vivo gene therapy works best for brain diseases caused by the lack of secreted protein: Transplanted cells can replace it by churning out the molecule for other neural cells to pick up. For many disorders, the missing protein operates within cells, and so all cells that need it must receive the vector.
(SOURCE) CLINICALTRIALS.GOV
The story of the viral vector that seems to answer this need begins with a death. In the early 2000s a leader of the field, geneticist James Wilson of the University of Pennsylvania (UPenn), was forced to redirect his research. Wilson had led the 1999 trial in which 18-year-old Jesse Gelsinger died from a massive immune reaction to a potent adenovirus vector that he had received to treat his liver disease. Wilson, who had a financial interest in the trial, faced a lawsuit by the family and an FDA investigation, and he eventually agreed to a 5-year ban on leading clinical trials. So he turned to finding new types of AAVs, which were safer than adenoviruses and had become a popular vector.
In 2004, Guangping Gao and others in Wilson’s lab reported that they had combed through human and primate tissues and found more than 100 new AAVs with a “tropism,” or preference, for infecting specific types of cells. One type, AAV9, was “unlike any other AAV. When you injected it into the blood at a high dose, it went everywhere”—heart, muscle, brain tissue—Wilson recalls. Most tantalizing of all was its ability to home in on neurons, which are the key to treating many brain and spinal diseases.
Other researchers scrambled to confirm AAV9’s love of the nervous system. In 2009, a French team and the lab of Brian Kaspar at Nationwide published separate papers that galvanized the field: Given intravenously to newborn mice, AAV9 crossed the blood-brain barrier—the tight network of cells that protects the central nervous system from pathogens and toxins—and infected neurons throughout the spinal cord and the brain.
When the Nationwide team was ready to try AAV9 in people, it chose to target SMA1, the most common genetic cause of death in babies. Infants with it lack a functioning SMN1 gene, which codes for a protein called survival motor neuron (SMN) that sustains spinal neurons throughout life. Most people have multiple copies of a backup gene, SMN2, that can produce small amounts of SMN, limiting the severity of the disease. But those with SMA1 have only two of those backup genes, and far too little of the crucial protein.
Babies born with SMA1 are floppy and can’t suck well or lift their heads; because spinal motor neurons are missing, their muscles weaken, and at some point they can no longer breathe on their own and die. A team led by Kaspar set out to develop a treatment by engineering AAV9 to carry the SMN gene. They planned to inject it intravenously, after showing that newborn mice with SMA1 that were treated in this way had normal motor function and life spans.
But that would likely require massive amounts of viral vector. Because liver cells are also a favorite target of AAVs, the liver removes most AAV9 injected into the blood; much of what remains goes to other tissues. As a result, less than 1% of the original dose crosses the blood-brain barrier, Gray estimated while he was a postdoc with UNC’s Jude Samulski. Based on the amount needed to treat SMA1 mice, the Nationwide team proposed a therapeutic dose of vector 100 times higher than had ever been injected into a patient’s blood in a gene therapy trial.
Yet tests with monkeys suggested the dose would be safe. FDA and the Recombinant DNA Advisory Committee, the National Institutes of Health (NIH) body that reviews most U.S. gene therapy trials, approved the study, which Nationwide and a foundation agreed to fund. (A new company co-founded by Kaspar called AveXis funded the later stages of the trial after licensing the therapy from Nationwide.)
Still, many gene therapy researchers held their breath, fearing another Gelsinger-like disaster. “It had a number of people nervous. It was a tremendous viral load being given to very young patients,” Samulski says.
Soon after the first baby received a test dose of the AAV9 therapy, her liver enzymes soared to 31 times normal, signaling possible cell damage. “I didn’t sleep well that night,” Mendell says. But steroids quickly brought the levels down, and FDA advised him to keep going, he says. Three other infants developed elevated liver enzymes, but there were no clinical signs of liver damage and the trial continued.

Although the trial was mainly designed to test the safety of the therapy, it quickly became clear that the introduced gene slowed the infants’ atrophy. Instead of becoming floppier and struggling to breathe, they got stronger. Lying on her stomach, Evelyn one day started to lift her head, Elena Villarreal recalls. “It was like, that’s it, that’s impossible with SMA1.” By now, only eight of the 15 treated babies need help from a face mask to breathe. And 11 of the 12 who received the larger of two AAV9 doses—one of whom was treated late, at 6 months—can sit at least briefly without help, eat, and speak. Matteo, a boy in Miami, Florida, who got the therapy just 27 days after birth, can run and is hitting normal developmental milestones, says his father, Derwin Almeida.
The results of the trial are “remarkable,” says Charlotte Sumner, an SMA researcher at Johns Hopkins University in Baltimore, Maryland. A drug called nusinersen, consisting of a strand of RNA that helps the SMN2 gene produce more protein, has had equally impressive effects in SMA1 infants in an ongoing study, and it was approved by FDA last December. But gene therapy is appealing because of the “one-and-done benefit,” Sumner notes.
How long the treatment will work is unknown. Unlike HIV and other viruses used for ex vivo gene therapy, AAVs don’t integrate the therapeutic gene into a cell’s genome; they deposit it as a free-floating loop of DNA, which means that the effects can be lost as cells copy themselves. But neurons don’t divide, so the treated tissue should keep producing SMN protein for years, says Kevin Flanigan, director of Nationwide’s gene therapy center.
If the gene therapy’s benefits eventually fade, the SMA1 patients would need retreatment, and they now have antibodies to AAV9 that might hamper it. But Nationwide researchers are working on countermeasures, such as filtering antibodies from the blood or giving certain immunosuppressing drugs, Mendell says.
Another distant worry is cancer. Studies by Sands and others have found that when given by IV in large doses to newborn mice, some AAVs can integrate their DNA into the genome and cause liver cancer. Sands notes that scientists don’t yet know whether those results are relevant to humans. Some gene therapists are reassured that liver cancer hasn’t appeared in the hundreds of patients exposed to AAVs in past gene therapy trials.
AveXis has launched a second SMA1 study, and it also plans to treat milder forms of the condition. If the trials bear out the promise of the therapy, producing enough doses to meet patients’ needs will be a challenge. But an AveXis spokesperson says a new plant near Chicago, Illinois, should have capacity to produce doses for the 500 SMA1 births each year in the United States and Europe. The company has not yet put a price tag on the treatment, but it will likely be expensive—gene therapies on the market command upward of $700,000 per treatment.
WEILL CORNELL MEDICINE BRAIN AND SPINE CENTER
Now that high-dose, systemically delivered AAV gene therapy appears safe, teams at Nationwide and elsewhere are launching trials for other neuromuscular diseases, which will aim to deliver a new gene by IV to muscle cells rather than neurons.
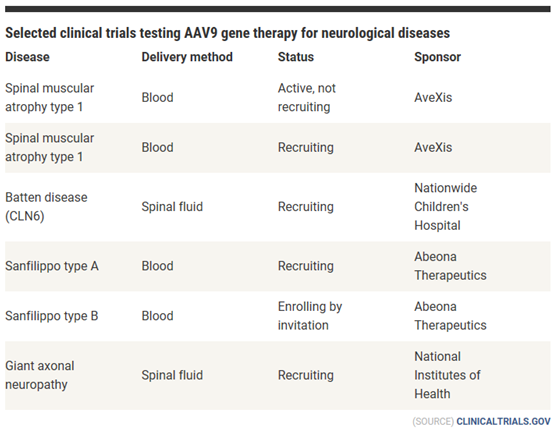
Scientists are also exploring the next question for AAV9: Can it go beyond helping spinal neurons and reach deep into the brain at levels high enough to halt diseases there? The first results could come from an ongoing trial, sponsored by the company Abeona Therapeutics and run by Nationwide, that is using IV delivery of an AAV9 gene therapy for Sanfilippo type A syndrome. Children with the disease lack an enzyme needed to break down heparan sulfate, a molecule that builds up in the brain and causes damage that is evident at ages 2 to 6. One year after three children were treated, levels of heparan sulfate in their spinal fluid have dropped sharply, their enlarged livers have shrunk, and their scores on nonverbal cognitive tests appear to have stabilized, Flanigan has reported at meetings.
Other trials rely on AAV9’s neuron-homing properties but administer it directly to a patient’s central nervous system through an injection into the spinal fluid (see table). This requires fewer viruses than IV delivery, so manufacturing won’t be as much of an obstacle; it also may work better for older patients, whose blood-brain barrier is more developed than infants’. And spinal delivery should reduce any immune response.
In the first test of this strategy, a trial at NIH has given eight patients with a disease called giant axonal neuropathy an AAV9 vector containing a replacement gene for the one at fault, which destroys axons—nerve cells’ impulse-transmitting tendrils—and affects the brain. Gray, whose animal studies in Samulski’s lab laid the groundwork for the trial, says it’s too early to report results. But Lori Sames of Rexford, New York, whose charity Hannah’s Hope Fund supported Gray’s research, says that her wheelchair-bound, 13-year-old daughter Hannah, treated in July 2016, has seen some improvements: Her vision loss has stabilized, and she can feed herself and stand with support. “We’re really happy with what we’re seeing,” Sames says.
For patients with SMA1 and other progressive disorders, early treatment is critical. “Once neurons are gone, they’re gone,” says pediatric neurologist Jonathan Mink of the University of Rochester Medical Center in New York. For now, siblings of children already afflicted by these genetic diseases are the most likely to be identified early enough for preventive treatment. But patient groups are urging that diseases such as ALD and SMA be added to newborn screening tests, which could expand the therapy’s reach.
Some researchers remain skeptical that the new, less invasive ways of delivering AAV9 will reach enough brain cells. “I have my doubts that it’s going to be widely applicable to other disorders,” especially in older children, says Ronald Crystal of Weill Cornell Medicine in New York City, who has used skull delivery to treat Batten disease.
Other gene therapists are pinning their hopes on finding new AAV vectors that are even better at crossing the blood-brain barrier. A group at the California Institute of Technology in Pasadena has identified several, including an AAV variant that crosses the barrier 40 times more effectively than AAV9 in mice. Voyager Therapeutics in Cambridge, Massachusetts, says these vectors appear to work in monkeys, too, though those data remain unpublished.
As thrilled as gene therapists are with the SMA1 study that gave Evelyn’s parents their dancing little girl, they will watch anxiously to see whether the same strategy can save more children with severe genetic diseases. If it does, gene therapy’s place in medicine’s arsenal will be assured. Says Wilson: “This was not a modest improvement. This is a transformational change. That’s what we had always hoped gene therapy would be.”
News from Science, By Jocelyn KaiserNov. 1, 2017 , 5:00 PM
http://www.sciencemag.org/news/2017/11/gene-therapy-s-new-hope-neuron-targeting-virus-saving-infant-lives